| References: |
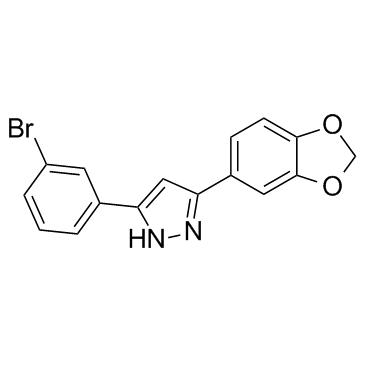
Oligomeric aggregates are presumed to be the key neurotoxic agent. Anle138b blocksthe formation of pathological aggregates of prion protein and of α-synuclein, which is deposited in Parkinson’s disease and other synucleinopathies such as dementia with Lewy bodies and multiple system atrophy. Anle138b strongly inhibits all prion strains tested including BSE-derived and human prions. Anle138b shows structure-dependent binding to pathological aggregates and strongly inhibits formation of pathological oligomers both for prion protein and α-synuclein.Anle138b has no detectable toxicity at therapeutic doses and an excellent oral bioavailability and blood–brain-barrier penetration. In mouse models of prion disease and in three different PD mouse models, anle138b strongly inhibits oligomer accumulation, neuronal degeneration, and disease progression. Di-phenyl-pyrazole anle138b binds to aggregated tau and inhibits tau aggregation. Anle138b treatment effectively ameliorates disease symptoms, increases survival time and improves cognition of tau transgenic PS19 mice. |