| References: |
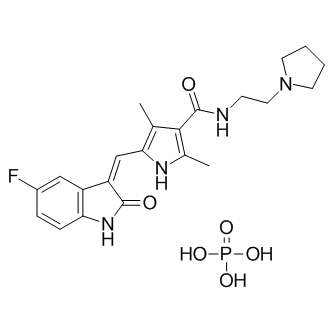
Toceranib ( Formerly known as SU11654) is a receptor tyrosine kinase inhibitor and is used in the treatment of canine mast cell tumor also called mastocytoma. Toceranib is the only dog-specific anti-cancer drug approved by the U.S. Food and Drug Administration. It is marketed as Palladia as its phosphate salt, toceranib phosphate (INN) by Pfizer. It was developed by SUGEN as SU11654, a sister compound to SU11248, which was later approved For human therapies as Sutent. Toceranib is likely to act mostly through inhibition of the kit tyrosine kinase, though it may also have an anti-angiogenic effect. For the detailed information of Toceranib(SU 11654; PHA 291639), the solubility of Toceranib(SU 11654; PHA 291639) in water, the solubility of Toceranib(SU 11654; PHA 291639) in DMSO, the solubility of Toceranib(SU 11654; PHA 291639) in PBS buffer, the animal experiment (test) of Toceranib(SU 11654; PHA 291639), the cell expriment (test) of Toceranib(SU 11654; PHA 291639), the in vivo, in vitro and clinical trial test of Toceranib(SU 11654; PHA 291639), the EC50, IC50,and Affinity of Toceranib(SU 11654; PHA 291639), Please contact DC Chemicals. |