| References: |
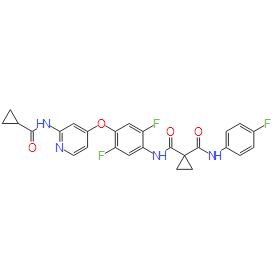
Altiratinib (DCC-2701) provided balanced inhibition of MET, TRK, TIE2 and VEGFR2 kinases. Altiratinib exhibited potency against both wild-type and mutant forms of MET and TRK kinases. In in vivo studies, altiratinib was shown to inhibit tumor growth, evasive vascularization, invasion and/or metastasis. In one model an increased overall survival was observed. Altiratinib exhibited anti-tumor activity in a variety of xenograft or allograft tumor models, including melanoma, gastric, lung, colorectal, breast, ovarian and glioblastoma. Altiratinib is currently in a Phase 1 clinical study in cancer patients with solid tumors.Altiratinib potently inhibited MET, TIE2, VEGFR2, and TRK kinases in functional cellular assays, including activity against proliferation, migration, and capillary tube formation, and with sufficient single-digit nanomolar potency such that all of these targets could be effectively inhibited simultaneously in vivo.Altiratinib exhibited efficacy at preventing tumor growth, as well as inhibiting evasive vascularization, pro-tumoral macrophages, epithelial-to-mesenchymal transition (EMT) and metastasis in a variety of cancer models.Altiratinib inhibited MET kinase for more than 24 hours after a single 10 mg/kg dose in a gastric cancer xenograft model leading to significant inhibition of tumor growth.Altiratinib blocked bevacizumab-induced evasive vascularization and EMT in an aggressive, invasive glioblastoma model.Altiratinib inhibited primary tumor growth and showed additive activity with paclitaxel; in addition, it reduced TIE2-expressing macrophages in the tumor stroma and significantly reduced lung metastases in a metastatic breast cancer model.Altiratinib exhibited a long off-rate from kinases (greater than 24 hours from TIE2 and TRKA) in a variety of cell-based assays, based on its binding mode.Altiratinib inhibited microvessel density and tumor growth in a xenograft model where both TIE2 and VEGFR2 kinases contribute to vessel growth.Altiratinib compared favorably with other multi-targeted MET inhibitors, and had additional activity in inhibiting oncogenic MET mutants found in papillary renal cell carcinoma (PRCC), while other MET inhibitors have not been shown to inhibit activated MET mutants. For the detailed information of Altiratinib(DCC-2701), the solubility of Altiratinib(DCC-2701) in water, the solubility of Altiratinib(DCC-2701) in DMSO, the solubility of Altiratinib(DCC-2701) in PBS buffer, the animal experiment (test) of Altiratinib(DCC-2701), the cell expriment (test) of Altiratinib(DCC-2701), the in vivo, in vitro and clinical trial test of Altiratinib(DCC-2701), the EC50, IC50,and affinity,of Altiratinib(DCC-2701), For the detailed information of Altiratinib(DCC-2701), the solubility of Altiratinib(DCC-2701) in water, the solubility of Altiratinib(DCC-2701) in DMSO, the solubility of Altiratinib(DCC-2701) in PBS buffer, the animal experiment (test) of Altiratinib(DCC-2701), the cell expriment (test) of Altiratinib(DCC-2701), the in vivo, in vitro and clinical trial test of Altiratinib(DCC-2701), the EC50, IC50,and affinity,of Altiratinib(DCC-2701), Please contact DC Chemicals. |