| References: |
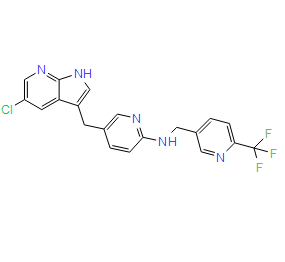
In the study, 23 patients with advanced PVNS in a single joint received 1000 mg of PLX3397 orally each day. Patients underwent an MRI every two months to measure tumor volume using a novel tumor volume score developed for PVNS. Among the 14 patients with evaluable MRI scans, the mean tumor size reduction was 61 percent. Eleven patients experienced a partial response (at least a 50 percent decrease in tumor volume compared to baseline screenings) and three had stable disease. Patients remained on the drug until disease progression or intolerability. For the detailed information of Pexidartinib(PLX3397), the solubility of Pexidartinib(PLX3397) in water, the solubility of Pexidartinib(PLX3397) in DMSO, the solubility of Pexidartinib(PLX3397) in PBS buffer, the animal experiment (test) of Pexidartinib(PLX3397), the cell expriment (test) of Pexidartinib(PLX3397), the in vivo, in vitro and clinical trial test of Pexidartinib(PLX3397), the EC50, IC50,and affinity,of Pexidartinib(PLX3397), For the detailed information of Pexidartinib(PLX3397), the solubility of Pexidartinib(PLX3397) in water, the solubility of Pexidartinib(PLX3397) in DMSO, the solubility of Pexidartinib(PLX3397) in PBS buffer, the animal experiment (test) of Pexidartinib(PLX3397), the cell expriment (test) of Pexidartinib(PLX3397), the in vivo, in vitro and clinical trial test of Pexidartinib(PLX3397), the EC50, IC50,and affinity,of Pexidartinib(PLX3397), Please contact DC Chemicals. |